PAMS Feature
Audit Trailing
CENTRALISED AUDIT OF ALL EVENTS & ACTIONS
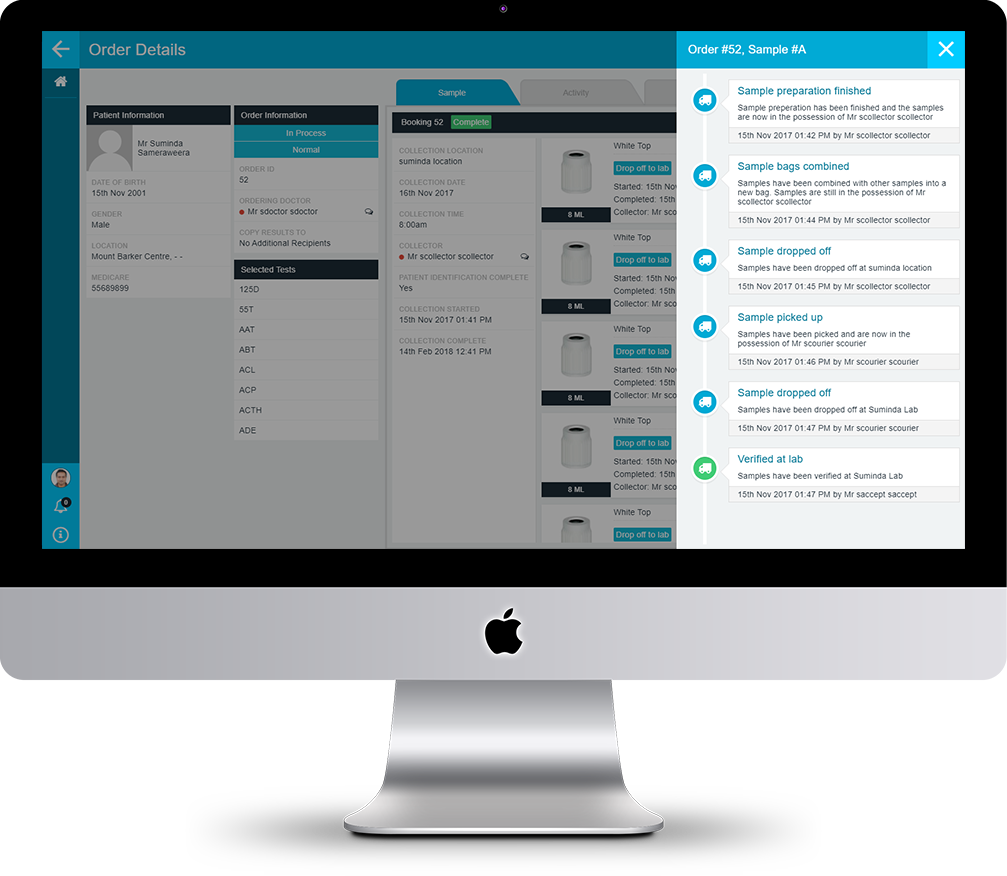
PAMS can audit every event and action that takes place throughout your pre-analytical process. Events can include user events, such as user logon or logoff, or actions such as positive identification methods used to identify a patient, collection of a sample, or receipt of an order. Although each user may be mobile and remote to the laboratory, PAMS maintains a central audit trail for every user and device connected to it. Using the audit trail, you have a complete and up-to-date history across every part of your key processes regardless of where each action is performed. PAMS provides you with logical views of the audit trail that make sense for each management function. For example, the order tracking view provides you with an order centric view where you can see every action relating to the processing of each order. Audit trail views, at a glance, give you a complete picture of everything that has taken place, allowing you to quickly pin-point the reason for issues or delays.
- Centrally maintained audit trail
- Audit all events and actions that take place
- Audit view for quick access and review

INTEGRATED EVENT AUDITING

As the PAMS audit trails is maintained as an integrated part of each process, there are no delays, procedural overheads or inefficiencies. Audit information is gathered unobtrusively and without distracting your staff from they core activities and procedures. Audit information is gather as a result of procedural actions or process steps to provide a consolidated view of every action and task, providing you with certainty and confidence that you have a complete record to meet management and compliance needs.
- Auditing events integrated within each process
- Centrally managed and accessible
- No procedural overheads or delays
CONFIGURABLE TO YOUR NEEDS
The PAMS audit trail can collect audit information regarding every event and action that that takes place within PAMS, but allows you to determine the exact information that should be maintained to meet your needs. Configuration options enable to refine and select the exact records that should be maintained for a given process (e.g. sample collections), for specific groups or roles (e.g. couriers, nurses) or for specific functions or actions (e.g. PPID compliance). You can determine your own audit needs and the level of audit information that you require to ensure that the audit information that you maintain is both efficient and effective.
- Central management & configuration
- Selectable & configurable audit events
- Target audit events for selected users & groups
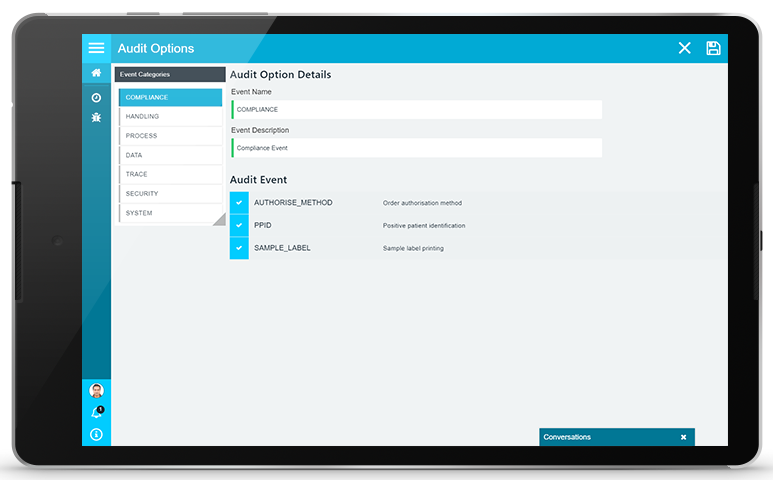
FULL COMPLIANCE AUDIT CONTROL
The PAMS audit trail maintains a complete record of all auditable events and procedures that are required to meet regulatory and corporate governance needs. Through audit trail and app configuration, you can refine or extend audit records to meet your specific compliance requirements.
- Integrated compliance records
- Define compliance audit events & requirements
- Central manageable & accessible compliance records
ANALYSE TRENDS, EFFICIENCY & PERFORMANCE
The PAMS audit trail can provide a wealth of information not only about when events happened, but also showing how and why events happened. PAMS provides you with a complete picture of every action and event that takes place throughout your pre-analytical process. PAMS provides a depth of information that has been difficult, if not impossible to gather, and with information comes knowledge and competitive advantage. Audit trail information may be used to analyse specific business elements such as clinician ordering patterns and patient trends, compliance performance, equipment usage and procedural efficiencies, collection centre performance.
- Analyse procedural and practice trends and performance
- Create analysis paths through data warehoused cubes and views
- Integrated data analysis tools