PAMS Feature
Positive Sample Identification (PSID)
PROCESS INTEGRATION
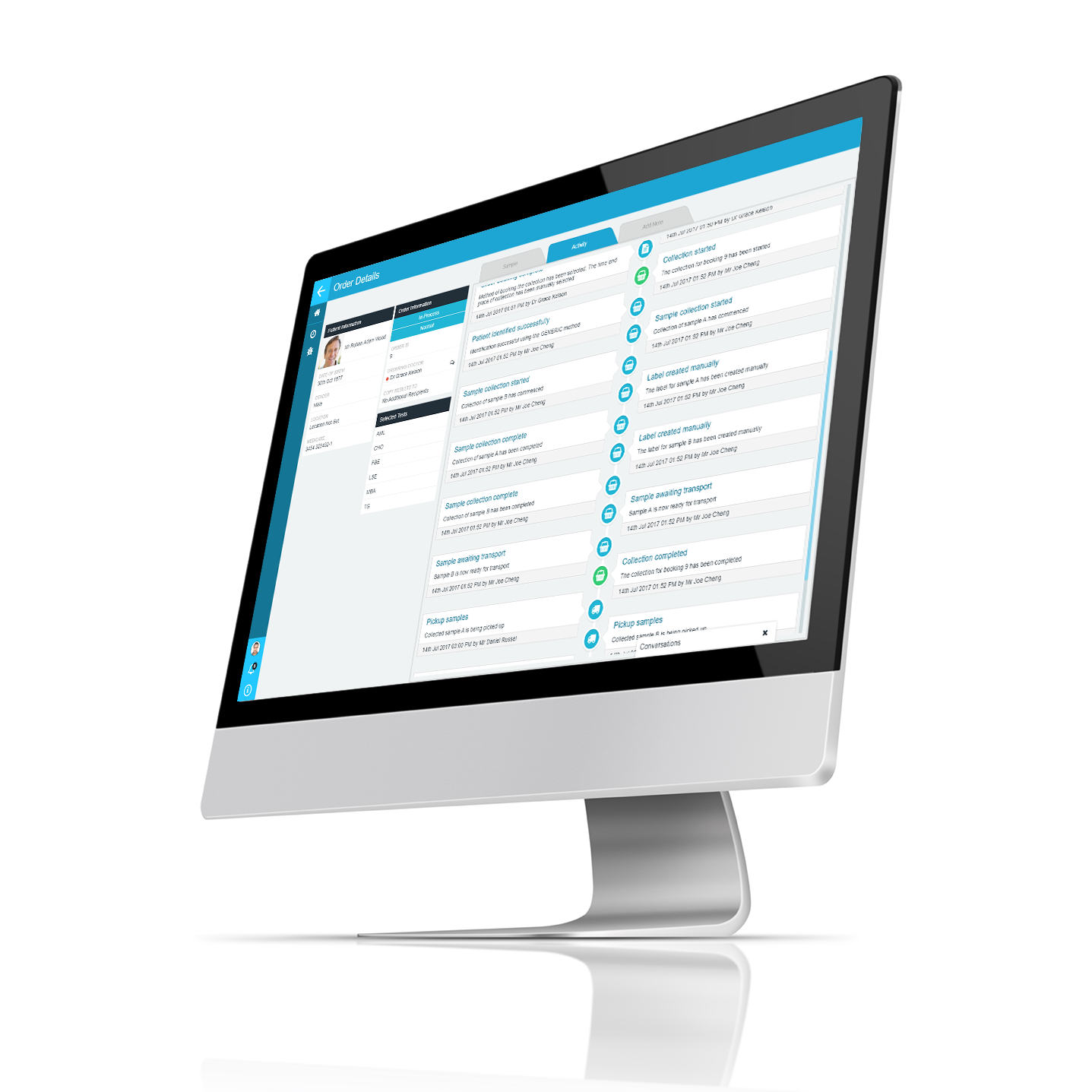
Incorrectly identified samples, or unidentified samples are once of the highest source of errors for labs. Errors pose a risk quality test results reporting and to patient safety. Even worse the possibility of “wrong blood in tube”, where the wrong patient is attributed to a sample. The onus is on the lab to identify and eliminate these samples before testing, and there are risks to patient safety and potential catastrophic adverse impacts should these errors not be detected. Failure can lead to, at least, loss of reputation for the lab and potential litigation. By integrating PAMS positive sample identification (PSID) into the collections app process, identification errors are eliminated before the sample reaches the lab. PAMS ensures that the right sample is identified for the right patient each time. Positive sample identification becomes an integral step within the collection process. By ensuring that each sample is correctly identified when collected and during the patient visit, the risk of mix-ups or identification errors is dramatically reduced. PAMS PSID ensures that each sample is fully and correctly identified before shipment to the lab.
- Integrated support for PSID best practices
- Full audit trail of all PSID events
- Definable rules and methods
- Add methods and PSID devices as required
- Centralised management
BEST PRACTICE IMPLEMENTATION
Using PAMS you can be sure that every time that positive sample identification is required, it is done in accordance with best practices. PAMS enables you to define the Positive Sample Identification (PSID) procedures and rules for each app, and being integrated, these rules may not be bypassed or skipped. The audit trail means that you will know the details of how and when each sample identified for every sample type. PAMS allows you to easily add new method and integrate PSID point of care devices as you need or as new technology becomes available, meaning that you PAMS implementation is always “future proof”.
- Integrated support for PSID best practices
- Full audit trail of all PSID events
- Definable rules and methods
- Add methods and PSID devices as required
- Centralised management
DEFINABLE RULES AND TEMPLATES
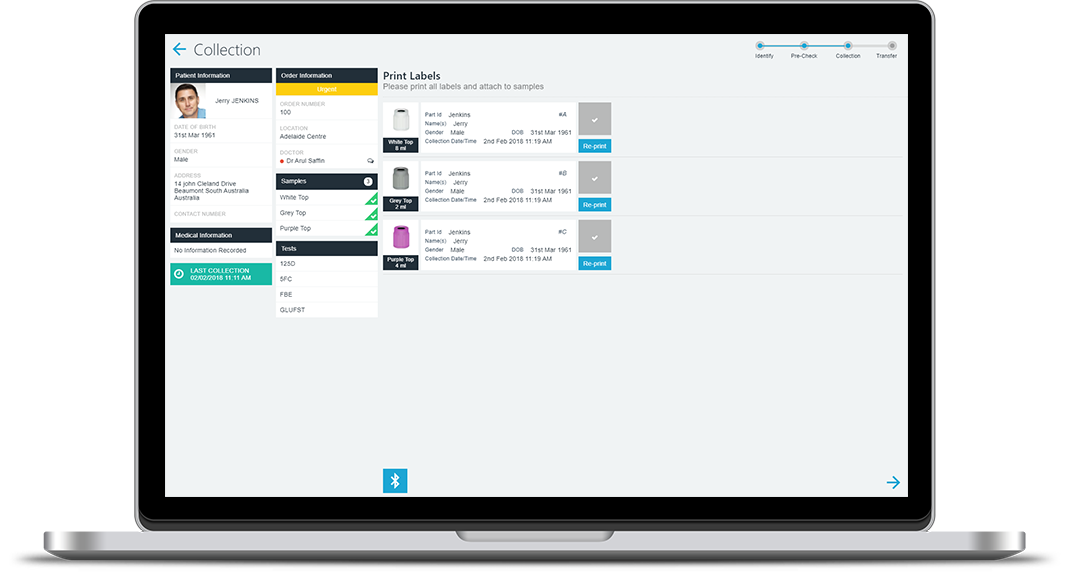
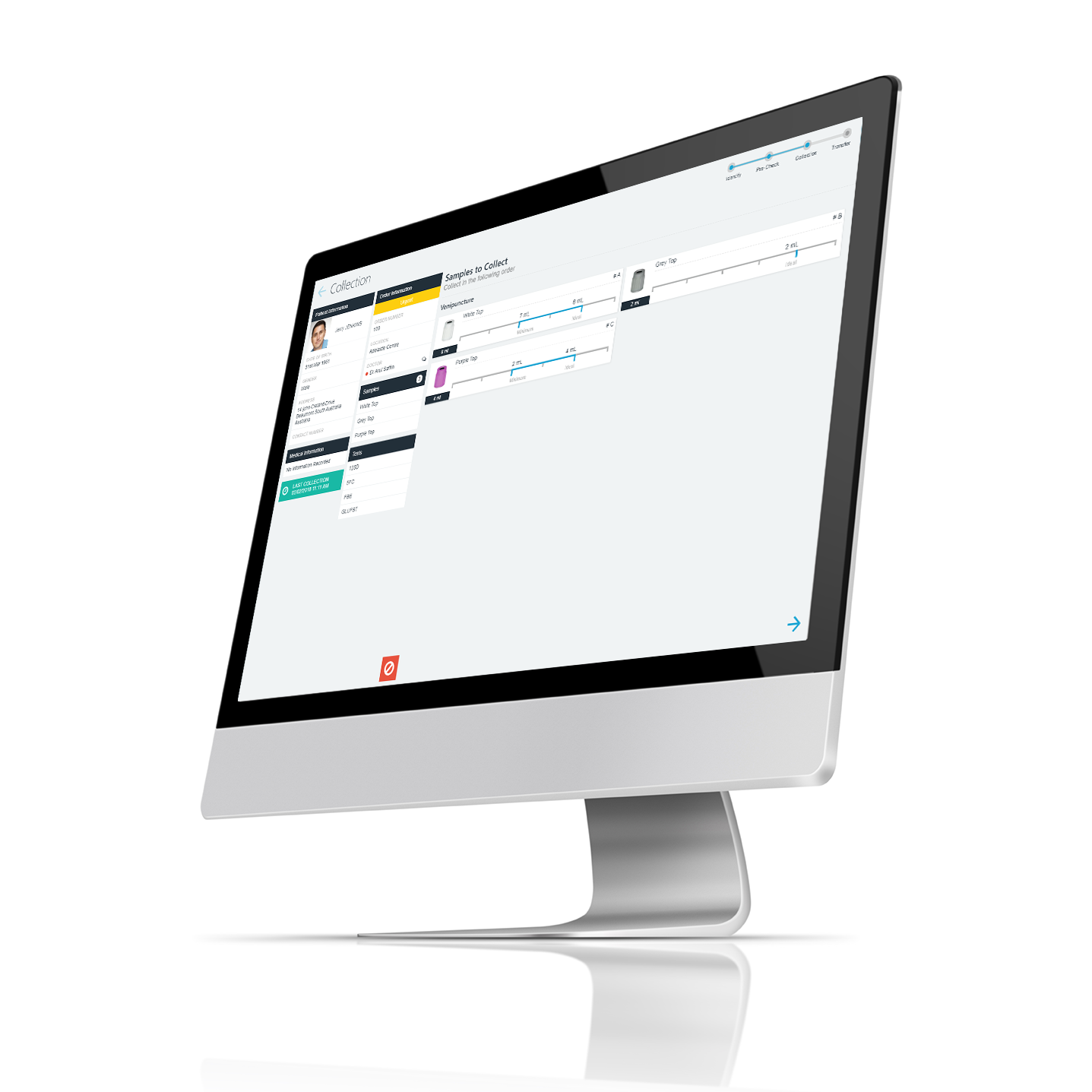
PAMS encourages and can enforce compliance with best-practice standards, but the rules that are applied are defined by you. Templates allow you to define the information and identification formatting that should be applied to each sample type. For example, where labels must be prepared, the lab can define a label template to include required patient and sample information as well as identification barcode(s) for processing. Once defined, each sample label produced uses the defined template. Rules may also be used to identification requirements that must be completed. You can change rules and add methods over time, and each PAMS app will be updated in line with your updated rules.
- Consistent rules applied across the organisation
- Define the forms of PSID to be used with each sample type
- Define mandatory requirements
- Define failure rules
- Integrated alerts and notifications
PLUG-IN METHOD SUPPORT
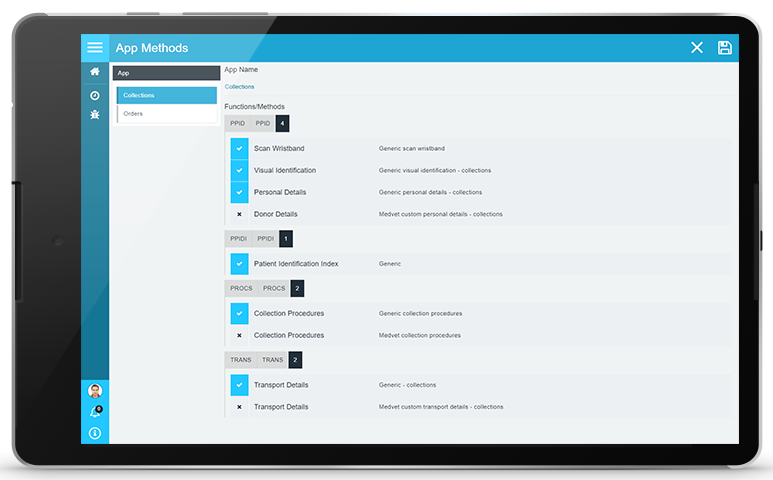
PAMS has been designed to respond to your changing needs, advancements in technology and changing best-practice standards. New methods for Positive Sample Identification can easily be added to PAMS apps though a centralised management centre. Once added, the new methods and associated rules will be automatically updated to every app user. This enables PAMS to be “future-proofed” and you can be confident that you will be able to stay in front of industry needs and technological advancements.
- Out-of-box PSID implemented methods
- Easy implementation of new methods
- Automated deployment of methods to all app users
POINT OF CARE IDENTIFICATION
Reduce the risk of mix up or labelling error by correctly identifying the samples during each patient collection episode. PAMS enables you to generate and complete sample identification at the time of the patient visit. No more pre-printing labels, PAMS uses mobile point-of-care devices to create sample identification on demand and as each sample is collected. Point of Care sample identification is integrated within the collection process to ensure that properly identified samples are always received at the lab.
- Eliminate the need for pre-printed labels
- Eliminate the chance of sample identification mix-ups
- Integrated sample identification with patient sample collection
